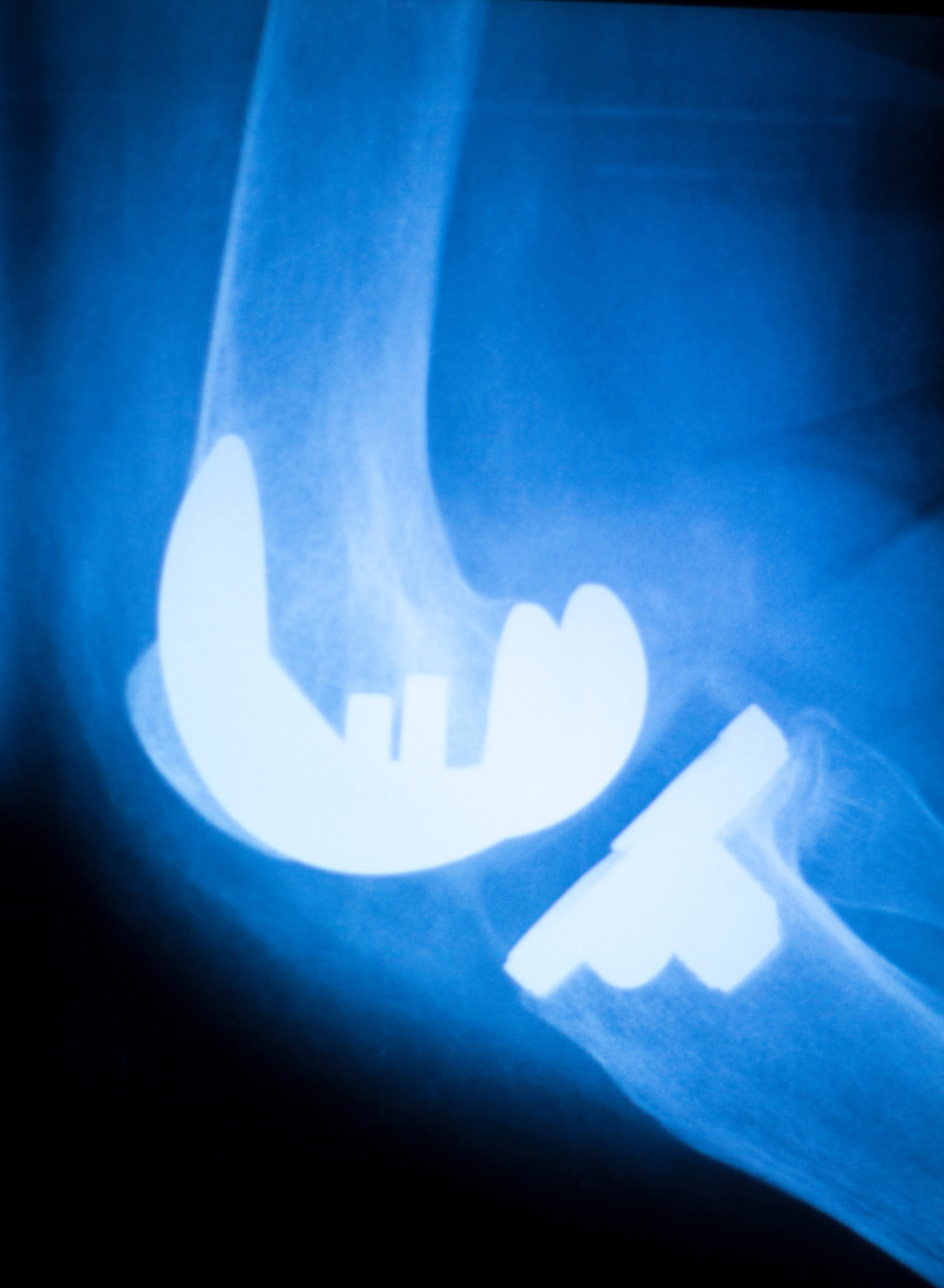
Exactech has recalled more than 148,000 Knee and Ankle Implants since February of 2022. It was reported that the polyethylene insert components and the potential for accelerated wear may crack causing the need for corrective revision or ankle surgery.
In addition to the recalled knee and ankle implants Exactech has recalled the Connexion GXL hip replacement liners.
Exactech notified the Food and Drug Administration (FDA) in July of 2021 that the polyethylene (plastic) was wearing out faster than expected in the implants.
Standards to meet for the recall:
Hip
- Implanted in surgery from 2008 – June of 2021,
- Patient had a revision surgery within 10 years of the original implanted device; or
- Claimant received a recall letter from Exactech; or
- Claimant was a patient of an Exactech doctor form the recall list or a patient of the orthopedic doctor on the recall list.
Knee
- Implanted in surgery from 2004 – February 2022
- Patient had a revision surgery within 10 years of the original implanted device; or
- Claimant received a recall letter from Exactech; or
- Claimant was a patient of an Exactech doctor form the recall list or a patient of the orthopedic doctor on the recall list.
Ankle
- Implanted in surgery from 2017 – February of 2022,
- Patient had a revision surgery within 10 years of the original implanted device; or
- Claimant received a recall letter from Exactech; or
- Claimant was a patient of an Exactech doctor form the recall list or a patient of the orthopedic doctor on the recall list.
If you or a loved one has suffered from injury or complications from a recalled Exactech Implant please call the experts at Inserra Kelley Sewell to see whether you might be entitled to compensation.