Medtronic Recalls MiniMed Insulin Pumps After Many Injuries and One Death
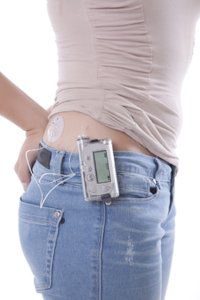 Medtronic is recalling over 322,005 of its insulin pumps because of a missing or broken retainer ring that can lead to over or under delivery of insulin. This can cause hypoglycemia or hyperglycemia in diabetics. The FDA has initiated a Class I Recall which means there may be significant or immediate danger of death or other serious injury from the product being recalled.
Medtronic is recalling over 322,005 of its insulin pumps because of a missing or broken retainer ring that can lead to over or under delivery of insulin. This can cause hypoglycemia or hyperglycemia in diabetics. The FDA has initiated a Class I Recall which means there may be significant or immediate danger of death or other serious injury from the product being recalled.
There have been over 26,421 complaints about the devices malfunctioning and one confirmed death. Over 2,175 injuries to diabetics that have used the MiniMed pump have occurred since the recall.
The recalled products are:
- MiniMed 600 Series Insulin Pumps
- Model 630G(MMT-1715) – all lots before October 2019
- Model 670G (MMT-1780) – all lots before August 2019
Consumers who have questions about this recall should call the 24-hour Medtronic hotline at 877-585-0166.
If you or a loved one has suffered from symptoms and/or injuries from using the Medtronic MiniMed insulin pumps, please call the experts at Inserra Kelley Sewell to see whether you might be entitled to compensation.



