Mibelas Nationwide Birth Control Recall
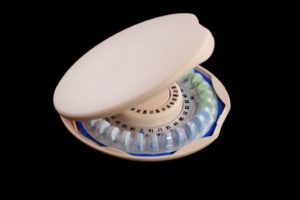 Lupin Pharmaceuticals, Inc. has voluntarily recalled some of its birth control pills, called Mibelas 24 Fe (Lot L600518, Expiration Date 05/18). A packaging error occurred, in at least one market reported complaint, in which the blister pack the pills are housed in was rotated 180 degrees so that the weekly tablet schedule was reversed and the wrong pills were taken at the wrong time. The first four days taken by the consumer were the placebos rather than the active pills. The result of such a mistake in manufacturing can be pregnancy. In addition to causing an unwanted pregnancy by such an error, the stakes could go even higher if an individual should become pregnant when pregnancy is contraindicated and even life threatening.
Lupin Pharmaceuticals, Inc. has voluntarily recalled some of its birth control pills, called Mibelas 24 Fe (Lot L600518, Expiration Date 05/18). A packaging error occurred, in at least one market reported complaint, in which the blister pack the pills are housed in was rotated 180 degrees so that the weekly tablet schedule was reversed and the wrong pills were taken at the wrong time. The first four days taken by the consumer were the placebos rather than the active pills. The result of such a mistake in manufacturing can be pregnancy. In addition to causing an unwanted pregnancy by such an error, the stakes could go even higher if an individual should become pregnant when pregnancy is contraindicated and even life threatening.
Customers with possible lot number of L600518 birth control pills, in a wallet of 28 tablets or carton of 3 wallets, are supposed to receive a recall letter. If in doubt, check the lot number on prescribed Mibelas pills to be safe. If you do find you have the affected lot, contact your personal doctor and take the pack back to your pharmacy immediately. The U.S. Food and Drug Administration (FDA) has a MedWatch program to which adverse events from such a product can be reported. The online version can be obtained at www.fda.gov/medwatch/report.htm, and the version to be downloaded can be obtained at www.fda.gov/MedWatch/getforms.htm.
If you or a loved one has already been adversely affected by Lupin’s Mibelas, or any medical device, drug, or other product, consult your medical provider immediately, and do not hesitate to contact Inserra Kelley Sewell, Personal Injury Attorneys, to discuss your possible claim and entitlement to compensation for your damages.

